3D Printing Combines with Medical Implant Design Software
Targeting medical, surgical, and orthopedic designers and manufacturers, C&A Tool and WITHIN have launched a patient-specific, metal-implant design and manufacturing program using EOS Direct Metal Laser-Sintering software to facilitate the creation of highly customized products that improve osseointegration or bone growth.
Posted: November 12, 2013
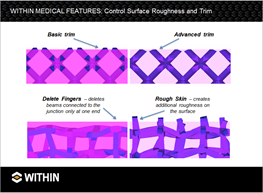
WITHIN Medical provides the user with complete control over the implant’s surface. Surfaces can be tuned (i.e. smooth, rough) based on application.
Finger implants can be made out of cobalt chrome or titanium using DMLS. These can be designed to allow varying levels of porosity to encourage bone in-growth into the implant structure.
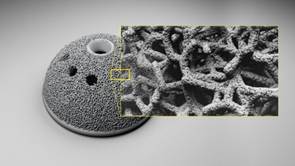
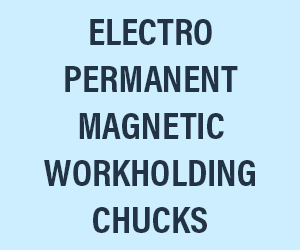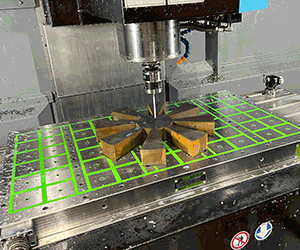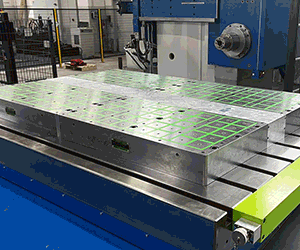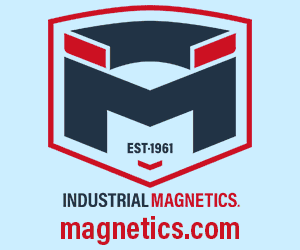
This hip implant design was created using WITHIN Medical software. The software works with plastic or DMLS systems to create strong parts, including innovative, lattice structures.
In a first-of-its-kind offering in North America, C&A Tool (Churubusco, IN) and WITHIN (New York City, NY) have launched a patient-specific, metal-implant design and manufacturing program called WITHIN Medical. The service combines free downloadable software developed by WITHIN, and additive manufacturing technology via Direct Metal Laser Sintering (DMLSTM) from EOS.
Targeting medical, surgical, and orthopedic designers and manufacturers, the software facilitates the creation of highly customized products that improve osseointegration or bone growth.
“With this program, companies now have the opportunity to explore the benefits that designing for DMLS can bring to their medical products—without investing in their own laser-sintering system,” says John Halverson, the medical business unit manager at C&A Tool. “Through this technology, they’ll be able to discover firsthand how DMLS reduces manufacturing steps, improves product performance and allows them to tailor medical products to individual patient needs.”
This training video is an overview of Within Medical v4.2, briefly covering all major features such as setting the properties of a part and lattice; adding surface regions; and creating variable density using kernels.
In traditional medical manufacturing the smooth, rough, and porous surfaces required for successful implantation are accomplished exclusively through secondary finishing — for instance, using machining along with plasma spraying or hand-sintering beads. DMLS eliminates many of these costly, additional processes by creating the necessary mix of surfaces as it builds the part layer-by-layer.
At the same time, the program offers engineers another benefit: the freedom to move beyond the limits of developing implants in predetermined sizes. Instead, they can choose to design patient-matched spinal reconstructions, acetabular cups, tibial trays, and other parts from MRI or CT scan data.
C&A Tool has complemented their extensive in-house machining capability with an investment in six EOS DMLS systems (both EOSINT M 270 and M 280). For orthopedic applications, the systems use biocompatible materials, such as medical-grade titanium (Ti64) cobalt-chrome, and stainless steel alloys.
The new software gives every designer the medium from which to manufacture highly complex geometries, including lightweight yet strong micro-latticed structures. EOS’s laser-sintering technology produces these intricate organic geometries as single-piece components.
Protecting proprietary digital models as they are passed between designers, OEMs, service bureaus, and contract manufacturers is also part of the new collaboration at C&A Tool and WITHIN.
“WITHIN Medical design software is free, easy to use and offers a high level of design control,” says Kaveh Mahdavi, a project manager at WITHIN. “When developing WITHIN Medical, we focused on the end-to-end solution, everything from design to manufacturing to validation. When coupled with additive manufacturing, the quality is superior to any existing products on the market.”
Highlights of the initiative that benefit medical manufacturers include no upfront investment for the user in either machine equipment or software, flexible business models, patient-specific products, design freedom and file encryption.
www.catool.com, www.withinlab.com